The research group of Guo Jing from the College of Chemistry published the research results in Nature Communications
Recently, the research group led by Guo Jing from the School of Chemistry published their research results in Nature Communications under the title "Visualizing alkali metal aggregation-induced coordination in CO2 activation on copper" [Nature Communications 16, 9463 (2025)].

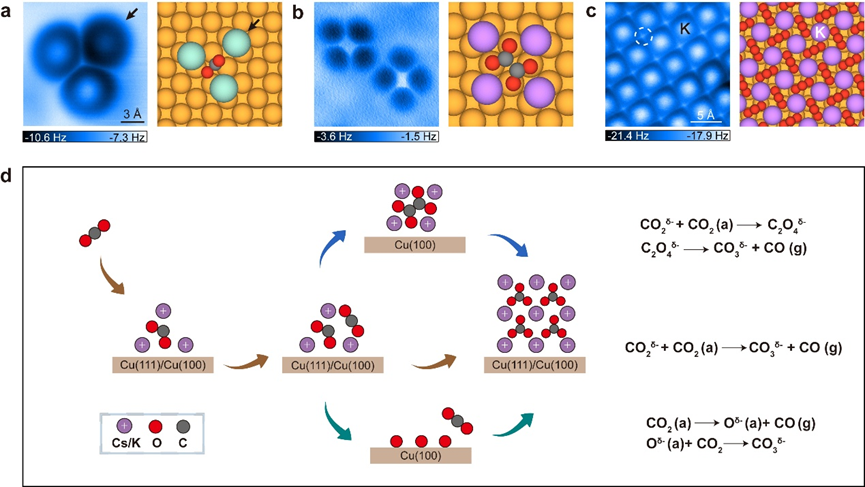
Abstract:
Alkali metals are widely recognized as promotors in CO2 activation and conversion. However, how the alkali metals activate CO2 molecules and stabilize the reaction intermediates remains controversial due to the lack of atomic-scale characterization. Here, using scanning tunneling microscopy and non-contact atomic force microscopy, we directly visualize the coordination structure of alkali metal cations (K+ and Cs+) and CO2 reaction intermediates on copper surfaces. At the initial step, we find the aggregation of alkali ions into trimers to facilitate the activation of CO2. Subsequently, the activated CO2δ- undergoes C-C coupling to form oxalate on Cu(100), that is coordinated with four alkali ions. Density functional theory calculations reveal the cooperative role of alkali trimers in stabilizing key intermediates, overcoming Coulomb repulsion, and significantly lowering the reaction barrier for CO2 conversion. Higher CO2 pressure promotes the production of two-dimensional ordered alkali carbonate films. Our findings provide valuable insights for designing efficient catalysts for carbon capture and utilization.
Reference: https://www.nature.com/articles/s41467-025-64499-4


