The Research Group Led by Professors MAO Lanqun and Wu Fei from the College of Chemistry Published Research Results in Nature Communications
Three-dimensional covalent organic frameworks (3D COFs) possess unique porosity and superior mass adsorption as well as transport capabilities over their two-dimensional counterparts (2D COFs), making them attractive materials for applications in catalysis, separation, and molecular recognition. However, the development of 3D COFs lags far behind 2D COFs and other 3D frameworks, largely hindered by bottlenecks in building block designs, synthesis and precise structure determination. To overcome these obstacles, it is essential to continuously explore new precursors underlying 3D COF topologies and related physiochemical properties.
Recently, the research group of Professors MAO Lanqun and Wu Fei from the School of Chemistry published a paper titled Steric effect of intermediates induces formation of three-dimensional covalent organic frameworks in Nature Communications.

The abstract of the paper is as follows:
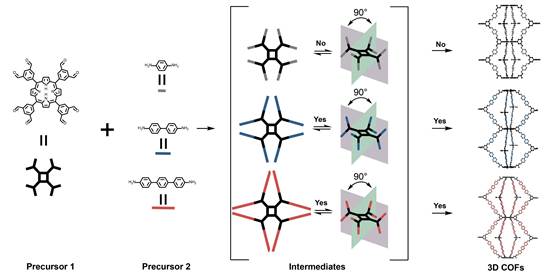
Constructing three-dimensional covalent organic frameworks (3D COFs) remains a significant challenge compared to other areas of reticular chemistry. Most existing methods for constructing 3D COFs emphasize the necessity of at least one precursor being extended into 3D space. As a result, designing and synthesizing precursors with specific stereoconformations has become a dominant strategy for discovering new 3D COFs. In this study, we leverage the principle that the steric hindrance of intermediates during COF formation is inherently greater than that of the precursors. We demonstrate a strategy utilizing multinode porphyrin blocks with eight rotatable knots to create intermediates with steric hindrance-confined rigid stereoconformations, which can be viewed as active precursors for guiding 3D formation of COFs with high crystallinity. This approach paves an avenue to self-adaptive COF growth towards diverse 3D topologies that may circumvent obstacles for traditional precursor synthesis.
Reference: https://www.nature.com/articles/s41467-025-61373-1


